Project I.D.E.F.I.X – EIC Transition 2022
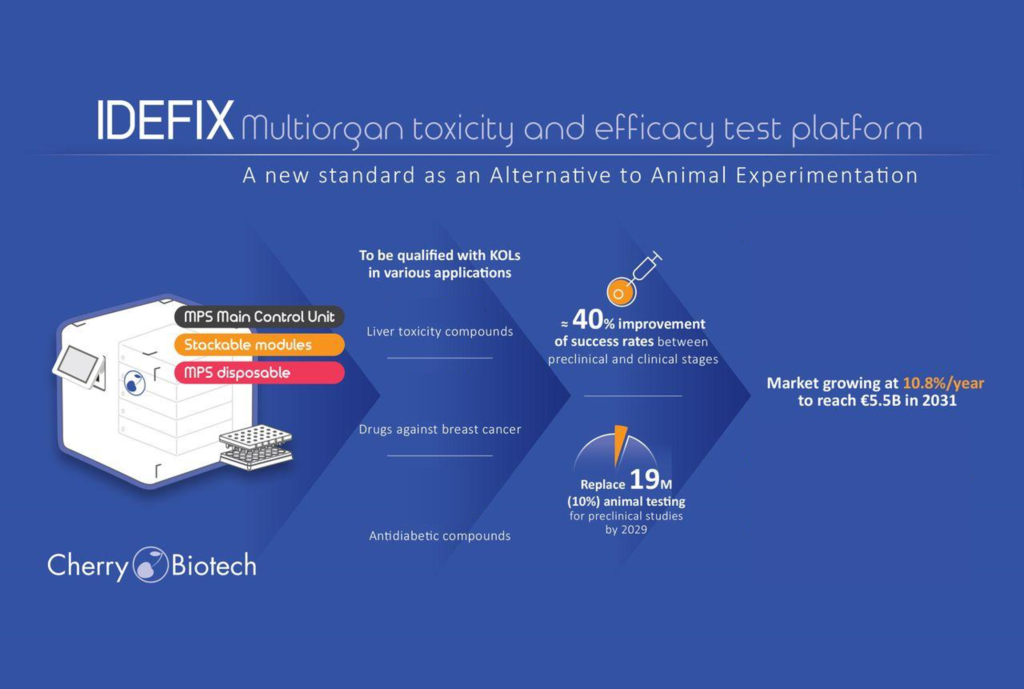
I.D.E.F.I.X project, a Multiorgan Toxicity and Efficacy Test Platform as a New Standard as an Alternative to Animal Experimentation
 AIMS
AIMS
Objective 1:
Develop the I.D.E.F.I.X. project platform from proof-of-concept (TRL3/4) to a viable demonstrator (TRL6)
Objective 2:
Qualify the new platform with known drugs in a relevant environment with industrial and academic end-users using three different applications: type 2 diabetes, liver toxicity, and breast cancer.
Objective 3:
Make our MPS Disposable prototype into a product.
 CHERRY’S WORK
CHERRY’S WORK
As project sole partner and leader, Cherry will implement the project. The IDEFIX project will run for 30 months to further develop, integrate, and validate the new microfluidic IDEFIX modular platform with external KOL partners. Divided into 4 interconnected work packages, its pathway to deployment is briefly described thereafter
Work Package 1: I.D.E.F.I.X. project Modular Platform Maturity Development
Work Package 2: Pre-Validation in-house and Qualification in Relevant Environments
Work Package 3: Go-to-market and business strategy preparation
Work Package 4: Management, IP, and dissemination
 EXPECTED RESULTS
EXPECTED RESULTS
- Design an architecture platform (hardware and software) integrating the previously developed stackable modules for Gas mixer, Flow, Temperature, Biosensing, Microscope & Imaging control.
- I.D.E.F.I.X. project platform demonstration and qualification in relevant industrial and hospital environments using respective biological models and known drugs
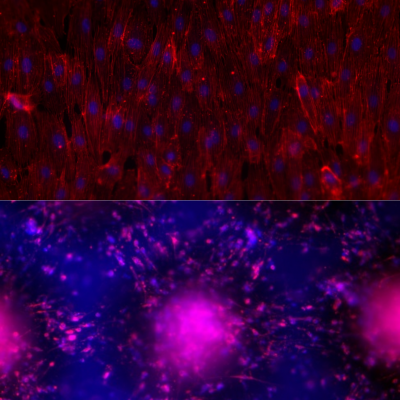
Top: Differentiated endothelium in 3D culture
Bottom: Organoids from metastatic breast cancer
 MANAGING TEAM
MANAGING TEAM

Antoni Homs Corbera
Responsible
WP1 Platform maturity development

Dario Fassini
Responsible
WP2 Biological validation and qualification

Alexis Peyroles
Responsible
WP3 Go to Market and Business strategy

Gaudriault Pierre
Responsible
WP4 Global Project coordination

This project has been funded by The European Union, under the grant agreement n° 101099775.
Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them
.

