Perfused Organs-on-a-Chip intergrated with TEER measurement technique:
A novel approach towards studying non-targeted drug induced organ toxicity
Organs-on-a-Chip model as an alternative for preclinical drug testing using animal models: State of the art and significance
There is growing global concern over the outbreak of new diseases and disease conditions. This makes it pertinent to synthesise new drugs to protect the society from any undesirable health conditions. The developmental process of a new drug molecule is cost-ineffective involving more than a billion Euros and particularly time-consuming taking about 10 to 15 years. In order to consider a newly synthesised drug molecule safe for human administration, the drug must undergo two stages, namely, preclinical and clinical trials in the drug developmental process. During the preclinical stages, the efficacy, toxicity and pharmacokinetics-pharmacodynamics (PK-PD) based data of the drugs are collected. It has been found that one out of ten drugs tested fail in the clinical trials owing to inefficient and unsuitable models available for the preclinical trial stage of the drug development process [1].
Conventionally, the preclinical stage in the drug developmental process involves implementation of two techniques namely, in vitro cell culture assay and in vivo animal experimentation. The preliminary in vitro cell culture assays performed using human-derived cells, facilitate to understand the cytotoxic effect of a chosen drug molecule [2]. However, the 2D cell culture assay that involves culturing of human-derived cells in petri dishes and multi-well plates does not mimic the physiological environment as the cells suffer from incomplete maturation, in addition, also lack complete functional development due to their confinement and growth in 2D space. Most importantly, 2D cell culture assay lack the organ-organ or tissue-tissue interaction and as a result cannot predict the complex drug metabolism and the effect of metabolite activity on non-target tissues [1,3]. Use of suitable animal models to test the in vivo drug efficacy and toxicity is another generally used method in the preclinical stage of drug development. Performing drug efficacy and toxicity experiments on animals take into consideration the multi-organ interaction and non-target organ toxicity. Nevertheless, there are ethical concerns over using animals. The use of animals also generates erroneous pharmacological data owing to the inaccuracies involved in extrapolating the results obtained due to species differences. Additionally, performing animal tests involves longer experimentation time and cost-ineffective [1-3].
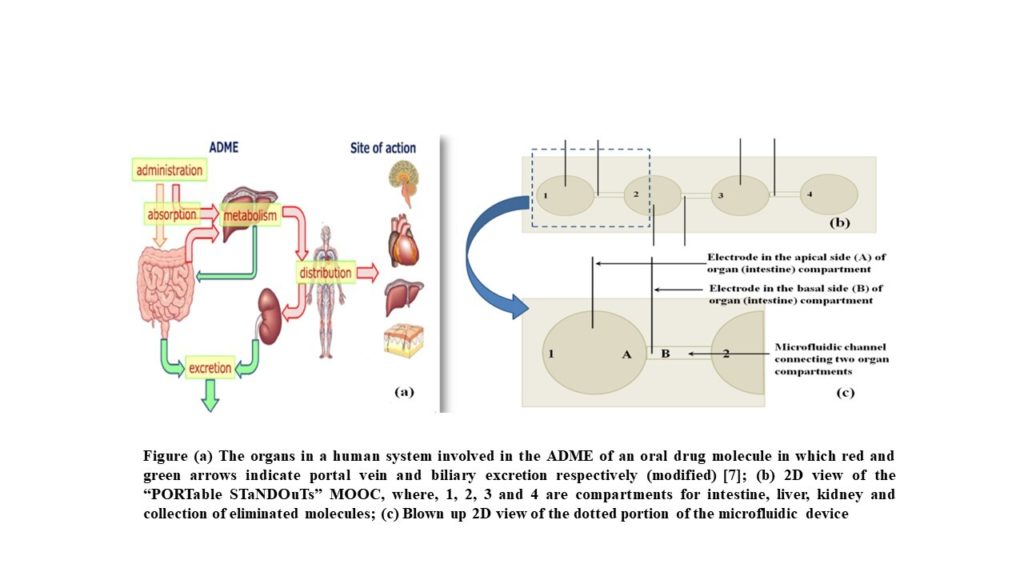
Therefore, a new emerging contemplation of fabrication of multiple Organs-on-a-Chip (MOOC) is gaining attention considering the aforementioned drawbacks of the existing in vitro and in vivo preclinical drug testing methods. An Organs-on-a-chip is a micro-engineered biomimetic device that contains microfluidic channels and chambers populated by living cells, which replicate key functional units of living organs to reconstitute integrated organ-level pathophysiology in vitro. In the European Union, it is believed that in vitro methods will play a major role in future legislation on testing chemicals and also in relation to the seventh amendment to the Cosmetic Directive. Thus, both of these policies demand for an alternative technique to replace animal experiments. Therefore, an extensive interest has been shown by many biotech, pharmaceutical, food and cosmetic industries in applying Organs-on-a-Chip for studying drug, nutrient and xenobiotic absorption and their possible toxic effects [4]. On administration of an oral pharmaceutical drug, major organs that are involved in the drug absorption and metabolism are intestine and liver. The drug is then distributed to different target organs such as brain, heart, lungs, etc. Finally, the drug metabolites are eliminated from the human system with the help of kidney.
Organs-on-a-Chip integrated with TEER measurement technique: Project insight
It is imperative to ensure that the microfluidic Organs-on-a-Chip mimic the physiological conditions as well as maintains the tissue uniformity and integrity. Human body is comprised of several barriers that separate the internal from the external environment as well as separate different compartments within the body. Such barriers that help in maintaining homeostasis by regulating the interactions between the different compartments are found in intestine, blood vessels, skin, lungs, brain and eyes. Disruption of such tissue barriers formed by cells constitutes the pathophysiological aspects of many diseases. Transepithelial electrical resistance (TEER) is the measurement of electrical resistance across a tissue monolayer and is a very sensitive and reliable method to confirm the integrity and permeability of the monolayer [5]. Conventionally, there are number of techniques that use a tracer compound to test the integrity of the tissue monolayer that provide qualitative insights into the barrier integrity of epithelial monolayer. They are immunostaining technique of proteins characteristic of tight junctions-integrated, freeze-fracture electron microscopy of transmembrane fibrils, permeability tests of the tissue monolayer/barrier to paracellular tracer compounds of various molecular weights. The use of radiolabelled markers have been to found to be sensitive technique, however, this technique require special safety precautions for handling and storage. Non-radioactive fluorescence labeled marker proteins have been found to be a suitable alternative for testing the monolayer integrity, considering the safety requirements needed while using radiolabelled markers. However, it has been found that the fluorescence labeled molecules, owing to either poor specific activity or fluorophore instability, do not provide the required sensitivity that can reflect the subtle changes in the tissue monolayer permeability. Also, the permeability assays involving fluorescent markers require bulky, complex and costly instrumentation as well as off-chip measurements [6]. Additionally, enzymatic markers are also being used to study the tissue monolayer integrity. However, the activity of the enzymatic markers was found to be affected mainly by factors such as pH, and temperature. Hence, in the conventional techniques, the use of such tracer compounds is found to interfere with the transport processes under study and to also affect the barrier integrity. In addition, the use of chemical dyes renders the monolayer unusable for further experiments. Thus, a non-invasive technique is indispensable to continuously monitor the tissue integrity [4]. TEER measurement is a sensitive, non-invasive, scalable and cost-effective technique that has the capability to enable real time monitoring of the integrity and permeability of the live tissue layer during various stages of growth, differentiation and experimental treatment [5].
The integration of TEER measurement with the Organs-on-a-Chip can be used to study parameters that control permeability and predict drug transport across these barriers in the early stages of drug discovery. Additionally, drug induced non-target organ toxicity caused by drug metabolites can also be studied using this TEER integrated Organs-on-a-Chip system.
Field of Applications
- Organs-on-a-Chip integrated with TEER measurement to ensure tissue integrity and uniformity
- Drug testing
- Non-target organ toxicity
Innovation and Future Applications
The development of a multi-parametric microfluidic Organs-on-a-chip toolkit with customizable layout in a high-throughput design will enable analysing various biochemical and physiological parameters such as drug response and toxicity concurrently using real-time TEER measurements. The proof-of-concept work will be to develop a three organs chip incorporating organ compartments that are involved in the absorption, distribution, metabolism and excretion (ADME) of an oral pharmaceutical drug namely, intestine, liver and kidney chip, with the provision for continuous monitoring of in-chip TEER values in real time to study the tissue uniformity and permeability that would in turn affirm the maintenance of physiologically mimicking conditions within the organ chambers throughout the experimentation. The developed chip will also be used to study the effect of typical drug molecules on the tissue integrity by carrying out real-time TEER measurements. Additionally, the application of the chip will be extended to study the non-target drug induced organ toxicity caused by metabolites produced by certain oral drug molecules. As an extended goal the developed chip would also be made versatile by incorporating other organs of interest that would be a positive contribution to the pharmaceutical drug development process phase.
References
- B. Esch, A. S. T. Smith, J-M Prot, C. Oleaga, J. J. Hickman and M. L. Schuler, Advanced Drug Delivery Reviews, 2014, 69-70, 158-169
- Kimura, Y. Sakai and T. Fujii, Drug Metabolism and Pharmacokinetics, 2018, 33, 43-48
- h. Sung, C. K and M. L. Shuler, Lab on a Chip, 2010, 10, 446-455
- Srinivasan, A. R. Kolli, M. B. Esch, H. E. Abaci, M. L. Shuler and J. J. Hickman, Journal of Laboratory Automation, 2015, 20, 107-126
- B. Arık, M. W. van der Helm, M. Odijk, L. I. Segerink, R. Passier, A. van den Berg and A. D. van der Meer, Biomicrofluidics, 2018, 12, 042218:1-13
- F. Wong and C. A. Simmons, Lab on a Chip, 2019, 19, 1060-1070
- Ishida, Drug Metabolism and Pharmacokinetics, 2018, 33, 49-54
 MANAGING TEAM
MANAGING TEAM

Dr. ANTONI HOMS CORBERA
Chief Technology Officer – Director of Technologies
Project supervisor

Dr. Divyasree Prabhakaran
R&D Manager (MSCA-IF)

This project has been funded by The European Union, under the grant agreement n° 884823.
Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.

