CubiX.
24 Multiwell plate mimicking physiological vascularized 3D adipose tissue for type 2 anti-diabetic during pre-clinical tox-efficacy studies
Why Cubix?
24 Multiwell plate mimicking physiological vascularized 3D adipose tissue for type 2 anti-diabetic during pre-clinical tox-efficacy studies
➨ PDMS Free
➨ Compatible with culture standard
➨ Incubator Free
➨ Vascularized 3D biological model
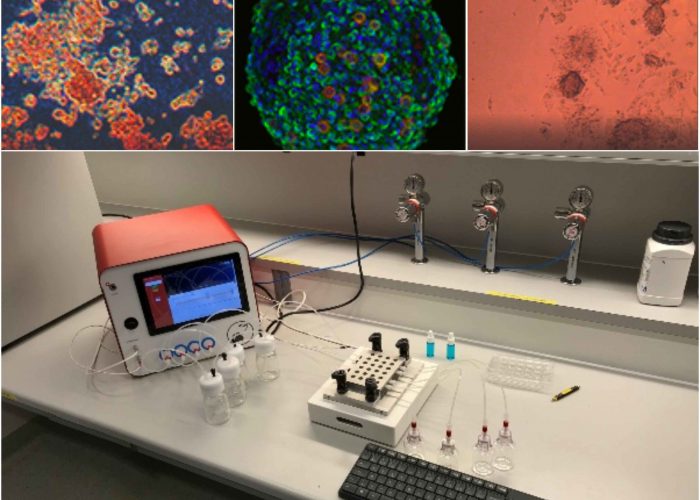



High Quality Research
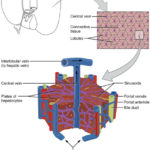
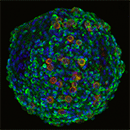
Obtain a functional 3D vascularized adipose tissue long term (> 2 weeks) for new molecular target identification and mechanism of action deciphering
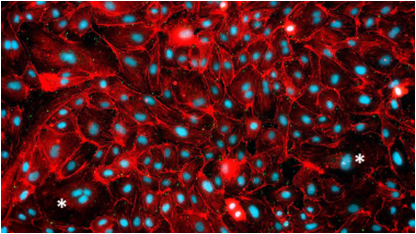
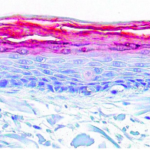
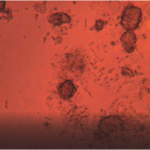
Static culture of endothelium cells, low alignment, and differentiation
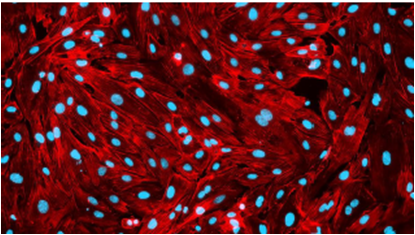
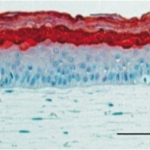
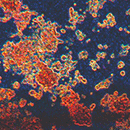
Cubix significantly increased alignment, differentiation, and integrity (tie junction)
Cherrybiotech
Get more out of your research
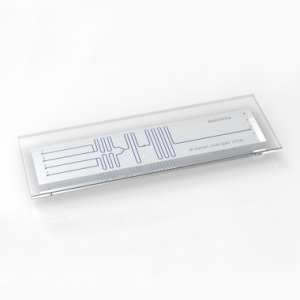
The only commercial MPS PDMS free device with no bias in toxicology and efficacy studies
Direct integration in existing analysis workflow, without disruption of the culture conditions
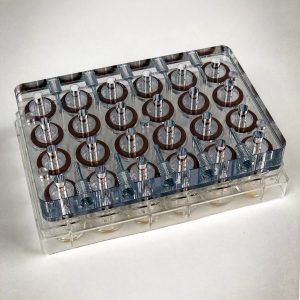
From 6 to 24 well allowing a direct transfer of the culture protocol
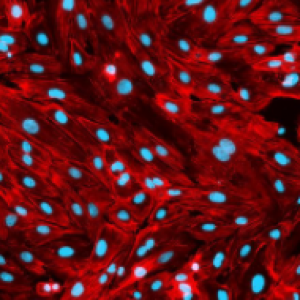
Physiological maintenance of a differentiated endothelium with an organ stroma >5 days
Testimonials
Learn how Cubix impacted researchers, the first incubator-free microenvironment controller in the world. Enabled the mimicking of physiological and pathological culture conditions in vitro. CubiX is the only microenvironment controller fully compatible with live imaging. You will never be blind to your sample ever.
CubiX is scalable & customizable allowing you to use custom made chips, multiwell, commercial microfluidic chip, and many more to help you get better and longer biological culture.




Discover the applications of Cubix
Our Partners