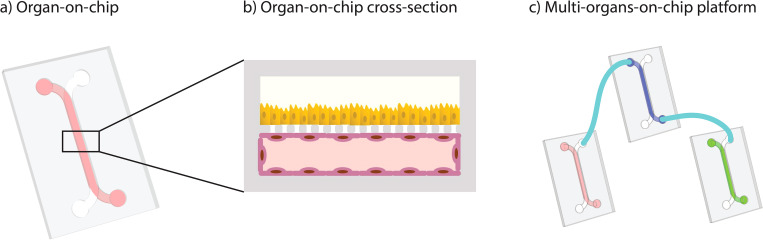
Facilitating implementation of organs-on-chips by open platform technology
Introduction Many Organ-on-Chip platforms are still not robust for all cell types, are not reproducible from experiment to experiment or user to user, and should be independently qualified as fit…